
Finally, Sweat has met its Patch

Finally, Sweat has met its Patch


Finally, Sweat has met its Patch
Candesant Biomedical, Inc. is a medical device company that has recently launched The Brella® SweatControl Patch.
Introducing targeted alkali thermolysis (TAT), a topical therapy for excessive sweating.
Introducing targeted alkali thermolysis (TAT), a topical therapy for excessive sweating.

Candesant’s mission is to offer an uncomplicated, effective alternative to current therapies to improve the lives of people who sweat too much. All 84 million of them.¹
Imagine "No Sweat" in Just Minutes




Our Technology: Targeted Alkali Thermolysis (TAT)
Focused delivery of heat through TAT, designed to inactivate the sweat glands.
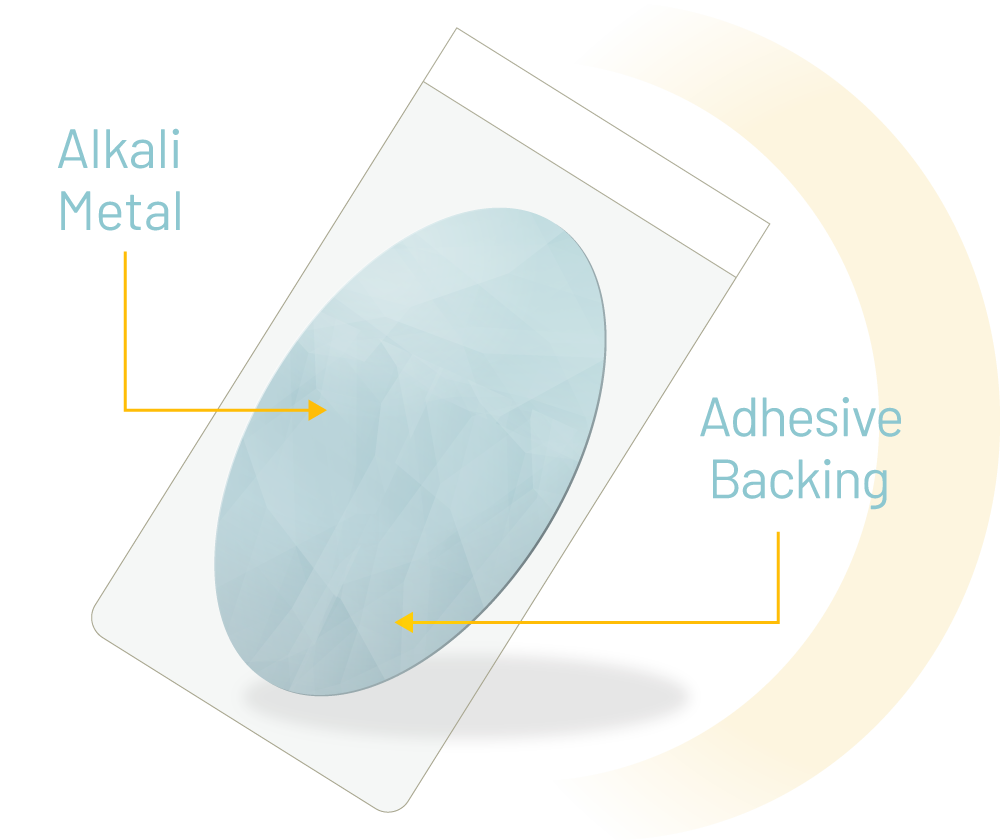
Interaction between alkali metals and water
Flexible, easy to use bi-layer patch

Desired outcome: reduced sweating
Excessive Sweaters Need More Solutions
Excessive sweating results in a severe quality of life impact with limited satisfaction of current treatment options
-
84 Million Excessive Sweaters¹
84M (about 1/3) of U.S. adults are bothered by their sweating and/or think they sweat too much under their arms.
-
15.3 Million Hyperhidrosis Sufferers²
15.3M (~5%) of the U.S. population suffers so much it's considered a disease state called hyperhidrosis.
Interested in learning more about what it means to sweat too much?
¹Strutton, et. al., “US Prevalence of Hyperhidrosis and Impact on Individuals with Axillary Hyperhidrosis: Results from a National Survey.” JAAD, August 2004, Volume 51, Number 2; Census Bureau, Population Division, 2015 Projections
²Doolittle, J., Walker, P., Mills, T. et al. Hyperhidrosis: an update on prevalence and severity in the United States. Arch Dermatol Res 308, 743–749 (2016). https://doi.org/10.1007/s00403-016-1697-9



